Introduction:
Venetoclax (ven) and azacitidine (aza) has become the standard of care for the treatment of patients (pts) with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy on the basis of the phase III VIALE-A trial, which showed prolonged overall survival with ven-aza vs. placebo-aza. Pts with active additional malignancies (AM) at the time of AML diagnosis are often unfit for intensive chemotherapy but are excluded from clinical trials, including VIALE-A. We aim to compare the characteristics and outcomes of AML patients with AM vs without AM (non-AM), all treated in a real world setting.
Methods:
A retrospective analysis including all AML pts diagnosed between Jan 2019 and Apr 2023 and treated with ven-aza in Tel Aviv Sourasky Medical Center. We defined active malignancy as any malignancy additional to AML that was diagnosed within 2 years prior to or concomitantly with the diagnosis of AML, or receiving active treatment at the time of the diagnosis of AML. We excluded prior MDS, MPN and basal cell or localized squamous cell carcinomas of the skin. We defined patients eligible for registration trial (RTE) as pts meeting the inclusion criteria for the VIALE-A trial. We compared categorical and continuous variables as appropriate, and examined survival outcomes by a Kaplan Meier and Log-Rank test. Event free survival (EFS) was defined as time until treatment failure (2 courses of ven-aza without achievement of response), relapse, or death.
Results:
We analyzed 113 pts diagnosed with AML and treated with ven-aza. 17 patients had an additional AM; 11 solid and 6 hematological, at the time of AML diagnosis. Six pts had more than one additional malignancy. Six patients had newly diagnosed AM, 6 had AM in remission and were treated with maintenance therapy at the time of AML diagnosis, and 5 had an active relapsed / progressive AM. Five patients received treatment for AM concomitantly or alternating with ven-aza, including surgery, letrozole, olaparib, arabiterone acetate, pazopanib, and trabectedin.
We compared the baseline characteristics and outcomes of AML pts with AM to AML pts treated with ven-aza who did not have AM (96 pts, non-AM) and pts who met inclusion criteria for the registration VIALE-A trial (57 pts, RTE). Pts with AM were younger than non-AM pts or RTE pts (11.8% over the age of 75 vs. 67% and 68%; respectively, p<0.001), and had a slightly higher baseline creatinine level than RTE pts (median=1.1 range 0.6-1.4 mg/dL vs. median=0.9 range 0.5-1.3 mg/dL; p=0.01). ECOG functional status, ELN risk category, and baseline cell counts were similar between all groups.
Treatment with ven-aza was initiated within a median of 6 days from diagnosis in AM pts (range 1-20). 58.2% of AM pts achieved CR/CRi vs. 57% of non-AM pts and 68% of RTE pts (p=0.9 and p=0.46, respectively). The 30 day mortality rate for AM pts was 17.6% vs 7.3% for non-AM pts (p=0.17) and 3.5% for RTE pts (p=0.07). Causes of early mortality among AM patients were non-responsive/progressive AML (2 pts) and sepsis (1 pt). Overall, 13 of 17 AM pts died, 7 from progressive AML, 3 from progressive additional malignancy and 3 from treatment complications. Six of the AM pts underwent allogeneic stem cell transplant (35%) vs. 14 non-AM pts (14.5%, p=0.07) and 11 RTE pts (19.2%, p=0.19).
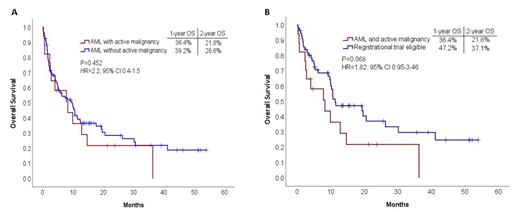
Median length of follow-up was 23.8 months (95% CI, 12.9-34.7). Overall survival (OS) EFS, and relapse free survival (RFS) were similar between AM and non-AM pts. (Figure 1A). ECOG score other than 0 (p=0.009, HR=2.2 95% CI 1.2-4.1) and secondary AML type (p=0.049, HR=1.8, 95% CI 1.001-3.124) but not AM (p=0.354, HR=1.42, 95% CI 0.67-3.03) were associated with reduced overall survival in a multivariate analysis which included age, sex and ELN risk category. A trend for worse survival outcomes in AM vs. RTE pts was noted (HR=1.82, CI 0.95-3.46 p = 0.068) (Figure 1B). EFS and RFS were similar between AM and RTE pts.
Conclusions:
Among pts diagnosed with AML and an additional active malignancy, treatment with ven-aza in the real world setting resulted in similar response and survival rates as in patients without active malignancies, but with a trend for worse overall survival and higher early mortality than pts who meet the eligibility criteria of the VIALE-A trial. 21.8% of AM pts were alive at 24 months. These results imply that treatment for AML for pts with active malignancy with ven-aza is feasible, and should not be withheld from these pts.
Disclosures
Moshe:Stemline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mittelman:Celgene/BMS: Other: participated in clinical trials, Research Funding, Speakers Bureau; MDS HUB: Consultancy; Media Digital: Speakers Bureau; FibroGen: Other: participated in clinical trials; Novartis: Other: participated in clinical trials, advisory boards, Research Funding, Speakers Bureau; Takeda: Other: participated in clinical trials, advisory boards; Astellas: Other: advisory boards; Gilead: Consultancy, Research Funding; Janssen: Research Funding; Roche: Research Funding; Silence: Other: advisory boards; Onconova: Other: advisory boards; Geron: Other: participated in clinical trials; Medison/Amgen: Research Funding; AbbVie: Other: participated in clinical trials, Research Funding. Avivi Mazza:AbbVie: Honoraria.